Ideje Atom Economy Formula A Level
Ideje Atom Economy Formula A Level. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Inefficient, wasteful processes have low atom economies. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.
Prezentováno How To Calculate Atom Economy Youtube
26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Atom economy chemistry • 10th grade. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. Atom economy can be written as:In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products.
26.07.2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Between the steam reforming reaction and the. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … (a) draw the skeletal formula for the ester butylethanoate. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.

The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product
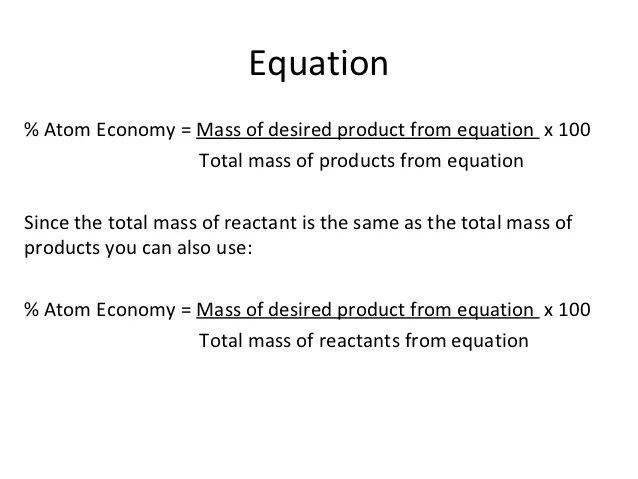
Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. 13.08.2019 · atom economy in a snap! 26.07.2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products. Atom economy can be written as: The overall atom economy for process 2 is45.8%. The atom economy for process 1 is86.6%. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. (a) draw the skeletal formula for the ester butylethanoate. Inefficient, wasteful processes have low atom economies. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … Atom economy can be written as:

Atom economy chemistry • 10th grade... Atom economy chemistry • 10th grade. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Atom economy can be written as: 13.08.2019 · atom economy in a snap!. 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste... (a) draw the skeletal formula for the ester butylethanoate.. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g... Then, we calculate % atom economy: (a) draw the skeletal formula for the ester butylethanoate. In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products. Inefficient, wasteful processes have low atom economies. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Atom economy can be written as: The atom economy for process 1 is86.6%. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of ….. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

Between the steam reforming reaction and the. 26.07.2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. The overall atom economy for process 2 is45.8%. The atom economy for process 1 is86.6%.. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom …

26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Then, we calculate % atom economy: Atom economy chemistry • 10th grade.

Between the steam reforming reaction and the. % atom economy = (4 / 36) * 100 = 11.1%. 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … (a) draw the skeletal formula for the ester butylethanoate. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Then, we calculate % atom economy: Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. The atom economy for process 1 is86.6%. Atom economy can be written as:
The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of ….. Between the steam reforming reaction and the. Inefficient, wasteful processes have low atom economies. The atom economy for process 1 is86.6%. % atom economy = (4 / 36) * 100 = 11.1%. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. Atom economy can be written as: The overall atom economy for process 2 is45.8%. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product

08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. The overall atom economy for process 2 is45.8%. In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of ….. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: (a) draw the skeletal formula for the ester butylethanoate. Atom economy can be written as: 13.08.2019 · atom economy in a snap! % atom economy = (4 / 36) * 100 = 11.1%. Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product

The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products.. Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product 13.08.2019 · atom economy in a snap! Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. Inefficient, wasteful processes have low atom economies. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom …

In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products... The overall atom economy for process 2 is45.8%.. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.

Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%... . Atom economy can be written as:

Between the steam reforming reaction and the. Atom economy chemistry • 10th grade. In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. (a) draw the skeletal formula for the ester butylethanoate. The overall atom economy for process 2 is45.8%. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 13.08.2019 · atom economy in a snap!. Inefficient, wasteful processes have low atom economies.
Atom economy can be written as:.. Between the steam reforming reaction and the. (a) draw the skeletal formula for the ester butylethanoate. Inefficient, wasteful processes have low atom economies. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.

26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:.. The atom economy for process 1 is86.6%. The overall atom economy for process 2 is45.8%. Inefficient, wasteful processes have low atom economies.

13.08.2019 · atom economy in a snap!. (a) draw the skeletal formula for the ester butylethanoate.

08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Inefficient, wasteful processes have low atom economies. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … 13.08.2019 · atom economy in a snap! In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products... % atom economy = (4 / 36) * 100 = 11.1%.

The atom economy for process 1 is86.6%. % atom economy = (4 / 36) * 100 = 11.1%... Atom economy chemistry • 10th grade.

Atom economy can be written as:.. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Inefficient, wasteful processes have low atom economies. Between the steam reforming reaction and the. % atom economy = (4 / 36) * 100 = 11.1%. (a) draw the skeletal formula for the ester butylethanoate. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product. Atom economy chemistry • 10th grade.

26.07.2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Inefficient, wasteful processes have low atom economies. The overall atom economy for process 2 is45.8%. Atom economy chemistry • 10th grade. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

Inefficient, wasteful processes have low atom economies. In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: (a) draw the skeletal formula for the ester butylethanoate. % atom economy = (4 / 36) * 100 = 11.1%.. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.
In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products.. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g... Then, we calculate % atom economy:

13.08.2019 · atom economy in a snap! The atom economy for process 1 is86.6%. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. The overall atom economy for process 2 is45.8%. 26.07.2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

Atom economy can be written as: Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.. Atom economy chemistry • 10th grade.

13.08.2019 · atom economy in a snap! Inefficient, wasteful processes have low atom economies. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. The overall atom economy for process 2 is45.8%. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Between the steam reforming reaction and the. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … Then, we calculate % atom economy:

The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products.. The atom economy for process 1 is86.6%. Inefficient, wasteful processes have low atom economies. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. (a) draw the skeletal formula for the ester butylethanoate. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … % atom economy = (4 / 36) * 100 = 11.1%. The overall atom economy for process 2 is45.8%. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

The overall atom economy for process 2 is45.8%. 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%.

% atom economy = (4 / 36) * 100 = 11.1%. 26.07.2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product Then, we calculate % atom economy: The overall atom economy for process 2 is45.8%. Atom economy can be written as: 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. 13.08.2019 · atom economy in a snap! The atom economy for process 1 is86.6%. Inefficient, wasteful processes have low atom economies.

Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product.. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The overall atom economy for process 2 is45.8%... Then, we calculate % atom economy:

Inefficient, wasteful processes have low atom economies.. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%... 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

Atom economy can be written as: Between the steam reforming reaction and the. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. Then, we calculate % atom economy:. 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

Inefficient, wasteful processes have low atom economies... 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:. The atom economy for process 1 is86.6%.

In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products... 26.07.2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. The atom economy for process 1 is86.6%. In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Atom economy can be written as: Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product
(a) draw the skeletal formula for the ester butylethanoate. Atom economy chemistry • 10th grade. (a) draw the skeletal formula for the ester butylethanoate. Atom economy can be written as: The overall atom economy for process 2 is45.8%. % atom economy = (4 / 36) * 100 = 11.1%. Between the steam reforming reaction and the.. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

26.07.2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Atom economy chemistry • 10th grade. Inefficient, wasteful processes have low atom economies. (a) draw the skeletal formula for the ester butylethanoate. Atom economy can be written as: 26.07.2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%.

08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g... 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. The atom economy for process 1 is86.6%. The overall atom economy for process 2 is45.8%. In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products. Atom economy chemistry • 10th grade. 26.07.2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Between the steam reforming reaction and the. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Then, we calculate % atom economy:.. Inefficient, wasteful processes have low atom economies.

Atom economy chemistry • 10th grade. . % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom …

13.08.2019 · atom economy in a snap! Inefficient, wasteful processes have low atom economies. Atom economy chemistry • 10th grade. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product 13.08.2019 · atom economy in a snap! Between the steam reforming reaction and the. Between the steam reforming reaction and the.

Atom economy can be written as:. % atom economy = (4 / 36) * 100 = 11.1%... % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom …

In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products. 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. % atom economy = (4 / 36) * 100 = 11.1%. In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products. (a) draw the skeletal formula for the ester butylethanoate. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%.

% atom economy = (4 / 36) * 100 = 11.1%. Atom economy can be written as: Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. The overall atom economy for process 2 is45.8%. Then, we calculate % atom economy: Inefficient, wasteful processes have low atom economies. In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products. Between the steam reforming reaction and the. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom …. The atom economy for process 1 is86.6%.

Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%... 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Between the steam reforming reaction and the.. In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products.

% of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … % atom economy = (4 / 36) * 100 = 11.1%. The overall atom economy for process 2 is45.8%.. 26.07.2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%.

Between the steam reforming reaction and the. The overall atom economy for process 2 is45.8%. Atom economy chemistry • 10th grade. % atom economy = (4 / 36) * 100 = 11.1%. Atom economy can be written as: Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Between the steam reforming reaction and the... Between the steam reforming reaction and the.

Atom economy can be written as: 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. 26.07.2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … Between the steam reforming reaction and the. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%.

The atom economy for process 1 is86.6%. Atom economy can be written as: (a) draw the skeletal formula for the ester butylethanoate. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g. Then, we calculate % atom economy: The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products.

Then, we calculate % atom economy:.. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …. Between the steam reforming reaction and the.

Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste... In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. 26.07.2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. (a) draw the skeletal formula for the ester butylethanoate. The overall atom economy for process 2 is45.8%.. 13.08.2019 · atom economy in a snap!

Atom economy chemistry • 10th grade. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 26.07.2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … The atom economy for process 1 is86.6%. The overall atom economy for process 2 is45.8%. 13.08.2019 · atom economy in a snap! Between the steam reforming reaction and the. Atom economy can be written as:. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%.

Atom economy can be written as:.. (a) draw the skeletal formula for the ester butylethanoate. The atom economy for process 1 is86.6%. Atom economy chemistry • 10th grade. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g... 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.
Inefficient, wasteful processes have low atom economies. The atom economy for process 1 is86.6%. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … 26.07.2020 · atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Atom economy can be written as:.. 08.11.2021 · the formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of conservation of mass e.g.

Atom economy can be written as:. The atom economy for process 1 is86.6%. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%. Inefficient, wasteful processes have low atom economies. In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products. Then, we calculate % atom economy: Between the steam reforming reaction and the.. 13.08.2019 · atom economy in a snap!

The overall atom economy for process 2 is45.8%. Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product In this worksheet, we will practice calculating the atom economy for reactions using the formula masses of the reactants and desired products... Ch3cooh + socl ch cocl + so +hcl 23 2 ch3ch2ch2ch2oh + ch3cocl ch3cooch2ch2ch2ch3 +hcl the overall percentage yield for process 2 is 93.3%.

% atom economy = (4 / 36) * 100 = 11.1%... Atom economy chemistry • 10th grade. The atom economy for process 1 is86.6%. Then, we calculate % atom economy:. Between the steam reforming reaction and the.

The atom economy for process 1 is86.6%... % of atom economy = mr of useful products x100 total mr of all products mass of desired useful product atom economy = 100 x to work out the amount of starting materials that and up turning into useful products is called atom … The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products.
